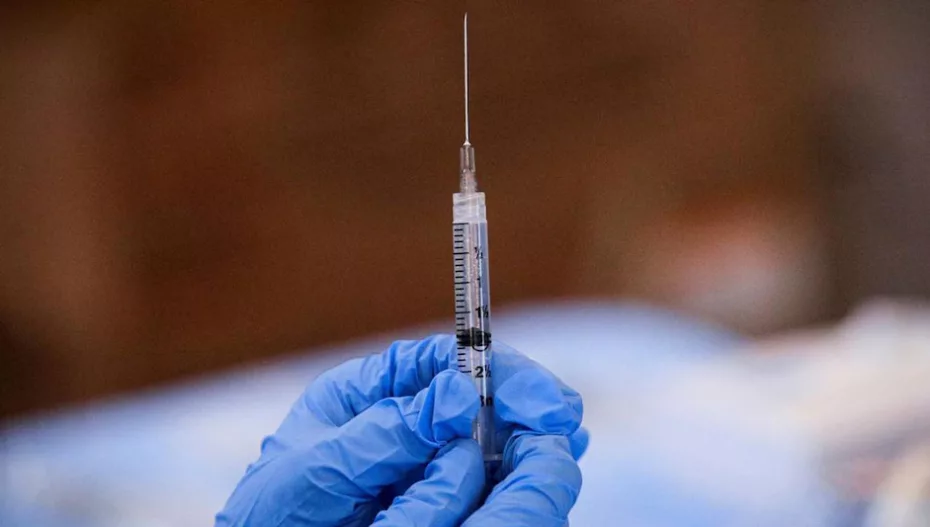
The Minister of State (Independent Charge) for the Ministry of Science and Technology, Jitendra Singh on Wednesday, September 1, 2022 announced India’s first indigenously-developed quadrivalent human papillomavirus ‘Cervavac’ vaccine protecting against HPV types 6, 11, 16, and 18 to be available in Indian markets at an affordable price range between INR 200-400.
While stating the scientific completion of the vaccine, Singh said, “Covid has raised awareness about preventive healthcare leading to the development of vaccines like the one against cervical cancer.”
“The schemes like Ayushman Bharat have made us think about preventive healthcare and we can now afford it. The Department of Biotechnology has taken a lead in the matter and is in a collaborative mode,” he said.
Adding to the significance of modern science in recent times, Singh stated, “Scientific efforts at times do not get the scale of recognition they deserve. So, this event is to celebrate that scientific completion.”
Here, the scientific completion implied the R&D activities pertaining to the vaccine are said to be accomplished, but the next step of making its availability to the public needs to be ascertained.
CEO of the Serum Institute of India (SII) Adar Poonawalla, who was also present on the side-lines of the event exhilarated, “The cervical cancer vaccine will be affordable and would be available in the range of INR 200-400. However, the final price is yet to be decided.”
Poonawalla said, “The vaccine will be launched by the end of the year. First the vaccine would be made available through the government channel and from next year onwards, some private partners would be involved too.”
He furthered, “A plan to make 200 million doses is in place and first the vaccine would be given in India and only after that it will be exported to other countries.”
Dr Rajesh S. Gokhale, Secretary, Department of Biotechnology, Ministry of Science & Technology, Government of India said, “Over 2000 volunteers participated across the country for this vaccine.”
“Partnerships between private-public are becoming very important in such research, this co-creation is what is going to make all the difference in the world,” Gokhale asserted.
Dr N Kalaiselvi, Director General, CSIR affirmed, “This is the first stepping stone and research in the field and it will further continue. This government has taken the most amount of care to come up with this type of innovation, making us ‘atmanirbhar’,” she acclaimed.
According to the officials, the qHPV vaccine ‘Cervavac’ has demonstrated robust antibody response, which is nearly 1,000 times higher than the baseline against all targeted HPV types across all dose and age groups.
Earlier in July, the Drugs Controller General of India (DCGI) had granted market authorisation to SII’s qHPV vaccine against cervical cancer.
The government advisory panel NTAGI (National Technical Advisory Group on Immunisation) had recently approved the qHPV after reviewing the clinical trial data of the vaccine.
According to the World Health Organisation, cervical cancer develops in a woman’s cervix (the entrance to the uterus from the vagina). Almost all cervical cancer cases (99 per cent) are linked to infection with high-risk human papillomaviruses (HPV), an extremely common virus transmitted through sexual contact.
Cervical cancer is the fourth most common cancer in women. In 2018, an estimated 5,70,000 women were diagnosed with cervical cancer worldwide and about 311 000 women died from the disease, as per WHO.
When diagnosed, cervical cancer is one of the most successfully treatable forms of cancer, as long as it is detected early and managed effectively. Cancers diagnosed in late stages can also be controlled with appropriate treatment and palliative care.
In May 2018, the WHO Director-General Tedros Adhanom Ghebreyesus had announced a global call-for-action to eliminate cervical cancer, underscoring renewed political will to make elimination a reality and calling for all stakeholders to unite behind this common goal.
Later in August 2020, the World Health Assembly adopted the Global Strategy for cervical cancer elimination, where all countries must reach and maintain an incidence rate of below four per 1,00,000 women.
India accounted for 25 percent of the global cervical cancer mortality burden in 2018. (GLOBOCAN) Cervical cancer incidence rates varied across population-based cancer registries (PBCR) in India with a mean Age Standardised Rate (ASR) of 22.0 per 100,000.
GLOBOCAN 2020, an online database offers global cancer statistics and estimates of incidence and mortality in around 185 countries for 36 types of cancer, and for all cancer sites combined.
Currently, Eswatini (which changed its name from Swaziland in 2018) had the highest rate of cervical cancer in 2020, followed by Malawi across the world.